【论著】| DDX6通过调控CKMT1A mRNA稳定性促进鼻咽癌细胞增殖和迁移的机制研究
2024-06-15 中国癌症杂志 中国癌症杂志 发表于上海
鼻咽癌细胞中DDX6通过破坏CKMT1A mRNA稳定性,负调控CKMT1A蛋白质翻译,增强鼻咽癌细胞增殖和迁移能力,从而促进鼻咽癌恶性进展。
[摘要] 背景与目的:DDX是一类腺苷三磷酸(adenosine triphosphate,ATP)依赖的RNA解旋酶,与mRNA调控、肿瘤增殖及侵袭等密切相关。本研究旨在探讨DDX家族成员DDX6对CKMT1A mRNA稳定性的影响以及DDX6-CKMT1A轴对人鼻咽癌细胞CNE2增殖和迁移能力的影响及其分子机制。方法:检索癌症基因组图谱(The Cancer Genome Atlas,TCGA)数据库中DDX6和CKMT1A在人头颈部鳞状细胞癌中的表达情况并进行相关性分析,利用蛋白质印迹法(Western blot)检测南通大学附属医院保存的人体鼻咽癌组织和正常鼻咽部组织中CKMT1A和DDX6的表达,本研究通过了南通大学附属医院伦理委员会的审查(编号:2022-L114)。利用transwell实验检测细胞迁移能力,采用EdU试剂盒检测细胞增殖能力,采用细胞集落形成实验检测克隆形成能力。转染慢病毒、质粒,构建南通大学附属医院保存的人源鼻咽癌细胞CNE2的sh-DDX6、sh-CKMT1A、sh-CKMT1A+sh-DDX6和oe-CKMT1A细胞模型,明确DDX6和CKMT1A表达水平对鼻咽癌细胞恶性生物学表型的影响。构建BALC/c裸小鼠皮下移植瘤模型,检测DDX6和CKMT1A在小鼠体内对鼻咽癌细胞成瘤性的影响。采用RNA稳定性实验检测敲除DDX6对CKMT1A mRNA的影响,进一步明确DDX6的分子机制。结果:人鼻咽癌组织中DDX6高表达,CKMT1A低表达,DDX6与CKMT1A表达呈负相关。DDX6通过破环CKMT1A mRNA稳定性,抑制CKMT1A蛋白质翻译。在CNE2细胞中,CKMT1A低表达可增强细胞迁移和增殖能力,高表达则抑制细胞迁移和增殖能力,而DDX6的敲除可逆转CKMT1A下调导致的恶性行为进展。在裸鼠皮下移植瘤模型中,低表达CKMT1A促进肿瘤细胞生长,低表达DDX6抑制肿瘤细胞生长,而同时敲除DDX6与CKMT1A能恢复单独敲低DDX6导致的抑制效果。结论:鼻咽癌细胞中DDX6通过破坏CKMT1A mRNA稳定性,负调控CKMT1A蛋白质翻译,增强鼻咽癌细胞增殖和迁移能力,从而促进鼻咽癌恶性进展。
[关键词] 鼻咽癌;CKMT1A;DDX6;细胞增殖;细胞迁移
[Abstract]Background and purpose: DDX is an adenosine triphosphate (ATP)-dependent RNA helicase closely related to mRNA regulation, tumor proliferation and invasion. This article aimed to explore the effect of DDX6, a member of the DDX family, on the stability of CKMT1A mRNA, as well as the effect of the DDX6 CKMT1A axis on the proliferation and migration ability of human nasopharyngeal carcinoma cell CNE2 and its molecular mechanism. Methods: We retrieved the data of expressions of DDX6 and CKMT1A in human head and neck squamous cell carcinoma from The Cancer Genome Atlas (TCGA) database and performed a correlation analysis. Western blot was performed to detect the expressions of CKMT1A and DDX6 in human nasopharyngeal carcinoma tissues and normal nasopharyngeal tissues preserved by Affiliated Hospital of Nantong University. This study was approved by the Ethics Committee of Affiliated Hospital of Nantong University (Number: 2022-L114). We used transwell assay to detect cell migration ability, EdU assay to detect cell proliferation ability, and colony formation assay to detect clone formation ability. We transfect with lentivirus and plasmids to construct sh-DDX6, sh-CKMT1A, sh-CKMT1A+sh-DDX6 and oe-CKMT1A cell models derived from the human nasopharyngeal carcinoma cell line CNE2, preserved by Affiliated Hospital of Nantong University, to clarify the impact of DDX6 and CKMT1A expression levels on the malignant biological phenotypes of nasopharyngeal carcinoma cells. BALB/c nude mice subcutaneous xenograft tumor model was constructed to detect the effects of DDX6 and CKMT1A on nasopharyngeal carcinoma cells in mice. RNA stability assay was used to detect the effect of DDX6 knockout on CKMT1A mRNA and further clarify the molecular mechanism of DDX6. Results: DDX6 was highly expressed, CKMT1A level was low in human nasopharyngeal carcinoma tissue, and DDX6 was negatively correlated with CKMT1A expression. DDX6 inhibited protein translation of CKMT1A by disrupting its mRNA stability. Low expression of CKMT1A in CNE2 cells enhanced cell migration and proliferation ability, while high expression inhibited migration and proliferation ability. Knocking out DDX6 reversed the progression of malignant behavior caused by downregulation of CKMT1A. Low expression of CKMT1A promoted tumor cell growth in BALB/c nude mice subcutaneous xenograft tumor model, while low expression of DDX6 inhibited tumor cell growth. Knocking out DDX6 and CKMT1A simultaneously restored the inhibitory effect caused by knocking down DDX6 alone. Conclusion: DDX6 in nasopharyngeal carcinoma cells disrupts the stability of CKMT1A mRNA, negatively regulates CKMT1A protein translation, upregulates the proliferation and migration ability of nasopharyngeal carcinoma cells, and promotes malignant progression of nasopharyngeal carcinoma.
[Key words] Nasopharyngeal carcinoma; CKMT1A; DDX6; Cell proliferation; Cell migration
鼻咽癌来源于鼻咽黏膜上皮细胞,是中国常见的头颈部恶性肿瘤之一[1],是发病率最高的头颈部鳞状细胞癌,常见于东南亚和中国南方地区。由于癌症发生的解剖位置隐蔽,早期症状不明显,很难发现,超过70%的患者初次就诊时已有淋巴结转移,20%~30%的患者最终会发生远处转移[2]。因此,深入研究鼻咽癌发病、转移的分子机制,对于鼻咽癌的防治具有重要意义。腺苷三磷酸(adenosine triphosphate,ATP)依赖的RNA解旋酶DDX6是RNA解旋酶家族成员之一[3],几乎参与RNA代谢的所有生物学过程,包括RNA转录、剪接、翻译、储存及降解等[4]。有研究[5]发现,DDX6在肺癌、胃癌、成神经细胞瘤、横纹肌肉瘤及结直肠癌等大多数恶性肿瘤细胞系中高表达。Maryam等[6]研究发现,DDX6为急性髓细胞性白血病细胞存活所需的转录后调节因子。DDX6参与多种肿瘤的发生、发展过程,但DDX6在鼻咽癌中的作用尚未见报道。CKMT1A位于线粒体膜表面,与细胞内能量传递、ATP再生直接相关,在肝癌[7]、非小细胞肺癌[8]等多种恶性肿瘤中高表达,但在胶质瘤[9]、前列腺癌[10]中的表达低于正常组织。既往研究[11]表明,过表达CKMT1A通过抑制STAT3磷酸化水平,从而抑制靶基因表达,提升鼻咽癌细胞对铂类化疗药物的敏感性。然而,CKMT1A对鼻咽癌细胞增殖和迁移能力的影响尚不清楚。DDX6对CKMT1A mRNA稳定性的影响也尚未见报道。
通过查询癌症基因组图谱(The Cancer Genome Atlas,TCGA)数据库发现,在头颈部鳞状细胞癌中DDX6与CKMT1A具有负相关关系,本研究拟进一步利用鼻咽癌组织及细胞分析DDX6与CKMT1A的表达水平及相关性,探究两者在鼻咽癌细胞中的具体调控机制。通过EdU试剂盒、transwell实验、集落形成实验、实时荧光定量聚合酶链反应(real-time fluorescence quantitative polymerase chain reaction,RTFQ-PCR)及RNA稳定性实验探索DDX6通过负调控CKMT1A蛋白质翻译,促进鼻咽癌细胞增殖和迁移的具体作用机制。通过小鼠体内实验探索DDX6和CKMT1A对鼻咽癌细胞的作用。
1 材料和方法
1.1 细胞和试剂
人源鼻咽癌细胞系CNE2来源于南通大学附属医院保存的细胞库。细胞用添加10%胎牛血清和100 U/mL青霉素-链霉素的RPMI-1640培养基在37 ℃、CO2体积分数为5%的恒温细胞培养箱中培养。RPMI-1640细胞培养基和胎牛血清均购自以色列Biological Industries公司,SV30010青霉素-链霉素购自美国HyClone公司,总RNA提取试剂(TRIzol)购自美国Invitrogen公司,兔抗人DDX6抗体、兔抗人CKMT1A抗体均购自武汉三鹰生物技术有限公司,二辛可宁酸(bicinchoninic acid,BCA)蛋白浓度测定试剂盒、反转录试剂盒和电化学发光(electrochemical luminescence,ECL)试剂均购自美国Thermo Fisher Scientific公司,GAPDH引物、DDX6引物和CKMT1A引物均购自南通翱翔生物技术有限公司,放线菌酮(Cycloheximide,CHX)、蛋白酶体抑制剂(MG132)和放线菌素D(Actinomycin D)均购自美国Monmouth Junction公司,EdU试剂盒购自上海雅酶生物医药科技有限公司,过表达CKMT1A慢病毒、敲低CKMT1A慢病毒和敲低DDX6慢病毒均购自上海吉凯基因医学科技股份有限公司。
1.2 方法
1.2.1 生物信息学分析
采用TCGA数据库下载并整理头颈部鳞状细胞癌项目STAR流程的RNAseq数据,整理出494例头颈鳞癌样本和44例正常组织样本,并提取FPKM格式的数据进行分析[12],数据处理方法为log2(value+1)。
1.2.2 人鼻咽癌组织
从南通大学附属医院保存的组织中选取了4例鼻咽癌组织和2例正常组织,用磷酸缓冲盐溶液(phosphate-buffered saline,PBS)润洗后,加入裂解液在冰上用研磨棒进行研磨,提取蛋白质。所有参与者在活组织检查前均未接受任何针对鼻咽癌的治疗,且均同意参与本研究,本研究通过了南通大学附属医院伦理委员会的审查(编号:2022-L114)。
1.2.3 转染
细胞接种于6孔板中,密度为50%~60%,培养至稳定,然后使用转染试剂聚凝胺转染慢病毒。转染具体方式按照操作说明进行。
1.2.4 蛋白质印迹法(Western blot)
细胞于放射免疫沉淀检测缓冲液中裂解,采用BCA试剂盒测定蛋白浓度,将30 μg蛋白样品加入梳孔中,进行十二烷基硫酸钠聚丙烯酰胺凝胶电泳(sodium dodecylsulphate polyacrylamide gel electrophoresis,SDS-PAGE),电转移至聚偏二氟乙烯(polyvinylidene fluoride,PVDF)膜上,并将膜泡在5%脱脂牛奶中封闭1 h,用浓度为1∶500的一抗稀释液温育4 ℃过夜,采用含有吐温-20三乙醇胺缓冲盐溶液(tris-buffered saline Tween,TBST)洗涤3次,再滴加浓度为1∶1 000的二抗稀释液室温温育1 h,采用TBST洗涤3次,避光下在蛋白条带上滴加ECL的发光液,压片后用显影液显影。
1.2.5 蛋白质稳定性
用10 μmol/L CHX和5 μmol/L MG132分别处理NC和sh-DDX6细胞,6 h后提取蛋白,用Western blot检测蛋白表达。另外在0、2、4、6、 8和12 h用10 μg/mL CHX处理NC和sh-DDX6细胞以阻止蛋白质合成,提取不同时间点的细胞蛋白,通过Western blot检测蛋白表达。实验重复3次。用Image J软件进行灰度分析。
1.2.6 RTFQ-PCR检测
采用TRIzol法提取细胞总RNA,使用反转录试剂盒得到cDNA,然后进行RTFQ-PCR。实验重复3次。CKMT1A、DDX6及内参GAPDH引物均由南通翱翔生物技术有限公司合成。CKMT1A的正向引物序列为5’-ATGGTGGCTGGAGATGAGGAGAC-3’,反向引物序列为5’-AGATCCGTGGTGTGCTTCAT TGTC-3’。DDX6的正向引物序列为5’-CCTCTG TGGCAAAGCGTGTCC-3’,反向引物序列为5’-CCCTTAATCCCAGCAACCTTCATCC-3’。各基因的表达通过GAPDH的表达而归一化。
1.2.7 mRNA稳定性
将5 μg/mL放线菌素D按指定时间点(0、2、4、6、8和12 h)加入细胞中抑制转录,收集RNA,进行RTFQ-PCR。实验重复3次。用ln2/slope法计算CKMT1A的半衰期,用GAPDH法进行归一化。
1.2.8 Transwell实验
将待测细胞培养至稳定,消化细胞,采用完全培养基终止消化,后用无血清RPMI-1640培养基重悬细胞于1.5 mL的离心管,采用细胞计数板计数,每个上室5×103个细胞,在24孔板中每孔加入500 μL完全培养基,上室加入200 μL细胞悬液,将24孔板继续培养在温育箱中约18 h。弃上室培养液,将小室浸润于多聚甲醛溶液中室温固定30 min,用结晶紫染液室温浸润30 min。用镊子取出小室清水冲洗,并用棉签轻柔擦拭,避免捅破膜,倒扣室温晾干,在显微镜下观察。实验重复3次。
1.2.9 细胞增殖实验
将细胞以每孔8×103个接种于96孔板中,在培养箱中继续温育18 h,按EdU试剂盒说明书操作,避光下通过荧光显微镜采集图片。实验重复3次。
1.2.10 细胞集落形成实验
将细胞以每孔100个接种于6孔板中,在培养箱中继续温育约2周至肉眼可见集落形成时,弃细胞培养液,在多聚甲醛溶液中固定15 min,用结晶紫染液染色15 min,冲洗干燥后采集图像。实验重复3次。
1.2.11 体内实验
从南通大学实验动物中心获得4~6周龄无胸腺免疫缺陷雄性BALB/c裸小鼠(每组5只)并通过动物伦理审核。每只小鼠皮下注射1×106个肿瘤细胞,每3 d观察小鼠生存状态及成瘤情况。皮下注射2周后,处死小鼠剥取肿瘤组织,并测量大小。用10%的甲醛溶液固定后制作成石蜡切片用作免疫组织化学(immunohistochemistry,IHC)检测。
1.3 统计学处理
使用GraphPad Prism 8.0软件对结果进行统计分析。两组之间的差异用t检验来确定,两个以上的群体之间的差异用单因素方差分析(one-way ANOVA)来检测。相关性分析使用皮尔逊相关系数。统计分析的详细信息用柱状图表示。 P<0.05为差异有统计学意义。
2 结 果
2.1 DDX6与CKMT1A在头颈部鳞状细胞癌组织中的表达呈负相关
通过检索、分析TCGA数据库,发现在头颈部鳞状细胞癌中DDX6高表达(图1A),CKMT1A低表达(图1B),且两者的表达具有负相关关系(图1C)。为证实这一发现,本研究使用Western blot分析了南通大学附属医院提供的4例鼻咽癌组织和2例正常鼻咽部组织标本中DDX6和CKMT1A蛋白的表达。与正常组织相比,4例鼻咽癌组织中DDX6蛋白表达水平较高,而CKMT1A蛋白表达水平较低(图1D)。
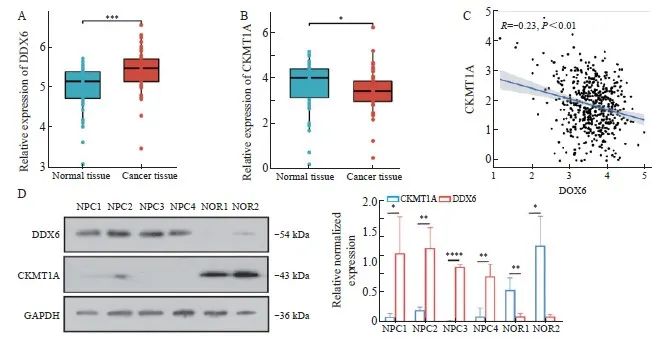
图1 DDX6和CKMT1A在鼻咽癌组织和正常鼻咽部组织中的表达
Fig. 1 Expression of DDX6 and CKMT1A in nasopharyngeal carcinoma tissues and normal nasopharyngeal tissues
A: Differential expression of DDX6 in cancer tissues and normal tissues in the TCGA database. B: Differential expression of CKMT1A in cancer tissues and normal tissues in the TCGA database. C: Pearson correlation analysis clarified the correlation between DDX6 and CKMT1A expression. D: Using Western blot to detect the expression of DDX6 and CKMT1A in nasopharyngeal carcinoma tissues and normal nasopharyngeal tissues. The right image is a quantitative image of grayscale analysis. NPC: Nasopharyngeal carcinoma patients; Nor: Normal rhinitis patients. *: P<0.05; **: P<0.01; ***:P<0.001; ****: P<0.000 1.
2.2 DDX6调控CKMT1A的mRNA稳定性
首先通过转染慢病毒在鼻咽癌细胞CNE2中构建DDX6敲低模型(图2A),并利用RTFQ-PCR和Western blot验证了敲低效率,与sh-DDX6-1组相比,sh-DDX6-2组的敲低效率更加显著,因此本研究选择sh-DDX6-2进行后续实验。用Actinomycin D在不同时间点处理NC和sh-DDX6细胞以阻断转录,提取RNA,通过RTFQ-PCR检测细胞中CKMT1A的mRNA表达水平,结果显示,DDX6的缺失显著增强了CNE2细胞中CKMT1A的mRNA稳定性(图2B)。进一步研究DDX6除了调节CKMT1A mRNA稳定性外,是否还可以调节CKMT1A的蛋白质表达,分别用MG132抑制蛋白酶体活性或用CHX阻断蛋白翻译。与NC组相比,sh-DDX6组细胞中CKMT1A的表达增多,在加入CHX处理6 h后,CKMT1A的表达皆受到抑制,而在MG132处理6 h的情况下蛋白质降解受到抑制,CKMT1A表达增强(图2C)。上述结果提示DDX6可能调控CKMT1A的蛋白质翻译。继续研究发现,在不同时间点加入CHX后,NC与sh-DDX6细胞之间CKMT1A的蛋白质半衰期没有显著差异(图2D),这更加证实了上述结论。
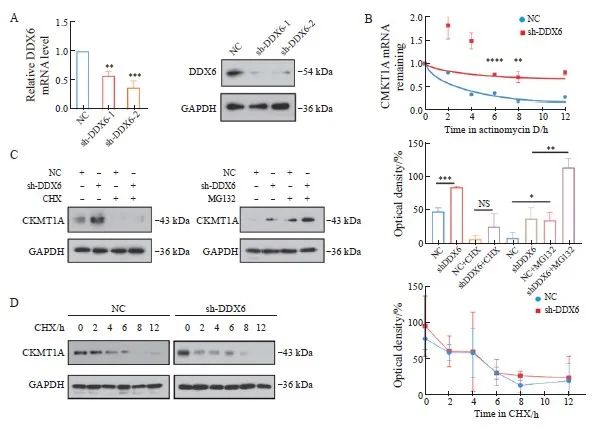
图2 敲除DDX6能够促进CKMT1A的mRNA稳定性
Fig. 2 Silencing of DDX6 can promote mRNA stability of CKMT1A
A: RTFQ-PCR and Western blot were used to verify the expression levels of DDX6 mRNA and protein after sh-DDX6. B: RTFQ-PCR was used to detect the mRNA expression of CKMT1A in cells treated with Actinomycin D at different time points. C: CHX (10 μmol/L) or MG132 (5 μmol/L) process NC and sh-DDX6 cells for 6 hours, detect the protein expression level of CKMT1A using Western blot (left), and perform quantitative analysis (right). D: Treat cells with 10 μmol/L of CHX at different times and Western blot was used to detect the protein expression level of CKMT1A (left) and quantitative analysis (right). *: P<0.05; **: P<0.01; ***:P<0.001; ****: P<0.000 1; NS: No significance.
2.3 CKMT1A抑制鼻咽癌细胞迁移、增殖和克隆形成能力
本研究构建了CKMT1A的过表达和敲低的细胞模型(图3A、3B),对CKMT1A在鼻咽癌细胞中的生物学作用进行初步探讨,通过测定mRNA和蛋白水平证实了CKMT1A的过表达及下调(图3B),sh-CKMT1A-3敲低效率更佳,因此选取sh-CKMTIA-3进行后续实验。利用transwell实验、EdU试剂盒及细胞克隆实验检测细胞迁移、增殖和克隆形成能力(图3C~3E)。检测结果显示,过表达CKMT1A抑制CNE2细胞迁移和增殖并减少肿瘤细胞的克隆形成数,与此相反,敲低CKMT1A可以明显促进CNE2细胞的恶性行为进展。为进一步验证上述结论,我们进行了BALB/c裸小鼠皮下移植瘤实验,2周后收取肿瘤组织,测量体积大小。与NC组相比,oe-CKMTIA组的肿瘤体积明显减小,而sh-CKMT1A组增大。IHC检测结果证实了肿瘤组织是否敲低或过表达CKMT1A(图3F、3G)。
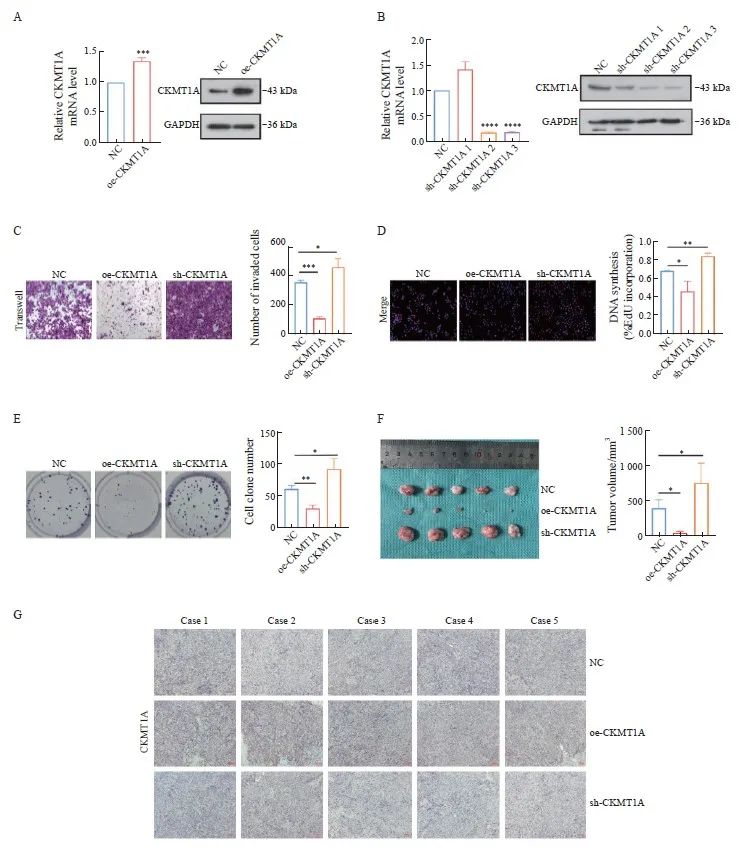
图3 体外和体内实验证实过表达CKMT1A可抑制鼻咽癌的恶性生物学行为
Fig.3 In vitro and in vivo experiments have confirmed that the overexpression of CKMT1A inhibits the malignant biological behavior of nasopharyngeal carcinoma
A: RTFQ-PCR and Western blot were used to verify the overexpression of CKMT1A by lentivirus. B: RTFQ-PCR and Western blot were used to verify the expression of CKMT1A after being knocked down by lentivirus. C: Transwell cell migration experiment, the bar chart shows the statistical data of the experiment. D: EdU cell proliferation experiment, the statistical chart is shown on the right. E: Cell clone formation experiment, quantitative image on the right. F: Subcutaneous tumor formation experiment in mice. Two weeks later, the tumor was collected and the volume was measured. The quantitative image is shown on the right. G: IHC staining was used to analyze the expression of CKMT1A in tumor tissue. Scale bar: 100 μm. *: P<0.05; **: P<0.01; ***: P<0.001; ****: P<0.000 1.
2.4 DDX6逆转CKMT1A对鼻咽癌细胞的抑制作用
为了证实DDX6能够逆转CKMT1A对鼻咽癌细胞的抑制作用,我们构建了sh-DDX6及sh-CKMT1A慢病毒转染细胞,用Western blot和RTFQ-PCR验证转染效率(图4A)。利用transwell实验、EdU试剂盒和细胞克隆形成实验证实了单独下调DDX6能够抑制CNE2细胞迁移、增殖和克隆形成能力,但当同时下调DDX6和CKMT1A则部分挽救了对CNE2细胞迁移、增殖和克隆形成能力的抑制(图4B~4D)。同时本研究构建了裸小鼠皮下移植瘤模型,敲低DDX6组明显小于NC组,且小于共同敲低DDX6和CKMT1A的肿瘤体积,更加证实了共同敲低组能够逆转敲低CKMT1A导致的恶性行为进展(图4E、4F)。
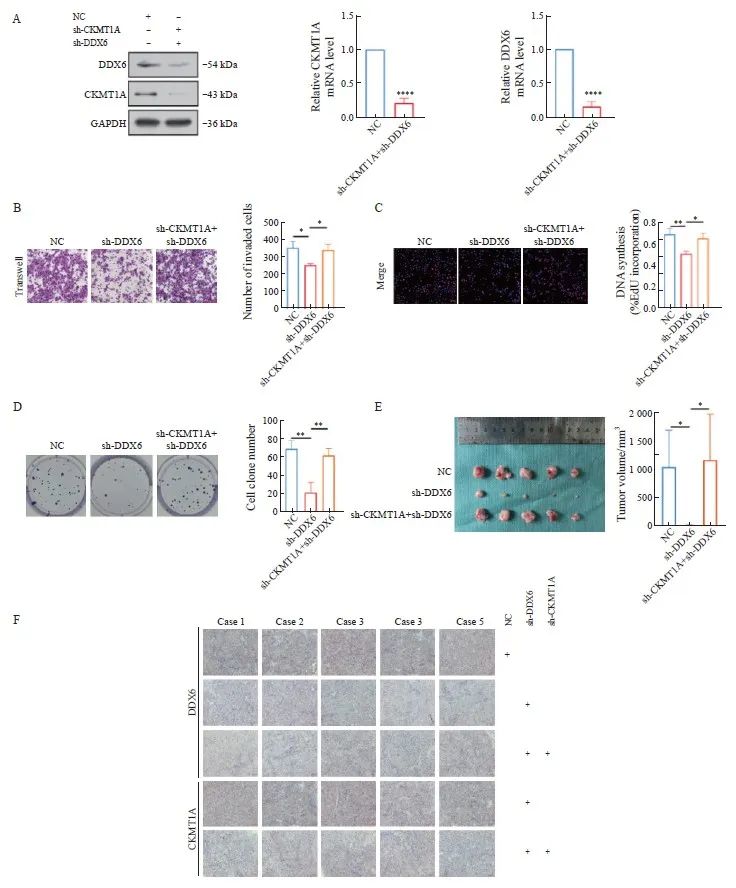
图4 下调DDX6可逆转下调CKMT1A对鼻咽癌的促进作用
Fig.4 Downregulation of DDX6 can reverse the promoting effect of downregulation of CKMT1A on nasopharyngeal carcinoma
A: The efficiency of simultaneous knockout of CKMT1A and DDX6 by lentivirus was verified by RTFQ-PCR and Western blot. B: Transwell cell migration experiment, the bar chart shows the statistical data of the experiment. C: EdU cell proliferation experiment, the statistical chart is shown on the right. D: Cell clone formation experiment, quantitative image on the right. E: The subcutaneous tumor formation experiment in nude mice was conducted. Two weeks later, the tumor was collected and the volume was measured. The quantitative image is shown below. F: IHC analysis of tumor DDX6 and CKMT1A expression. Scale bar: 100 μmol/L. *: P<0.05; **: P<0.01; ****: P<0.000 1.
3 讨 论
鼻咽癌是一种易复发和转移的恶性肿瘤,深入阐明鼻咽癌发病、转移的分子机制,对于鼻咽癌的防治具有重要意义。本研究结果表明,CKMT1A在鼻咽癌组织和细胞中低表达,DDX6高表达,DDX6通过破坏CKMT1A mRNA稳定性促进鼻咽癌细胞迁移和增殖。
CKMT1A是一种存在于线粒体内膜外表面的线粒体蛋白,它能够将磷酸盐从线粒体转移到细胞质载体来促进磷酸肌酸能量的跨线粒体转移[13]。CKMT1A在多数恶性肿瘤中高表达,但本研究发现其在鼻咽癌中是抑癌分子,另有研究[11]表明,过表达CKMT1A可以提升人鼻咽癌细胞对铂类化疗药物的敏感性,由此本研究旨在继续深入探索CKMT1A在鼻咽癌中的积极作用。DDX6几乎参与RNA代谢的全部过程,包括翻译起始、前mRNA拼接和mRNA降解,因此其会影响到RNA的生成和多态性,进而影响到蛋白质生成和细胞生物学表现[14]。有研究[15]表明,包括DDX6在内的多种RNA解旋酶,如DDX5、DDX17、DDX1及DDX3X的异常表达与癌症的发生息息相关,它们的作用被认为高度依赖于癌症类型。以往研究[16]表明,DDX6与血管内皮生长因子(vascular endothelial growth factor,VEGF)的内部核糖体进入位点结构相互作用,在缺氧的情况下,DDX6的降低有助于VEGF表达的激活并促进其血管生成功能。此外,DDX6蛋白通过与真核起始因子4E(eukaryotic initiation factor 4E,eIF4E)相互作用作为翻译的监督协调者,表明DDX6可能通过参与调节细胞增殖基因的翻译和启动而促进细胞生长,这是一种新定义的致癌机制[17]。国外研究[18]发现,DDX6正向影响Warburg效应,这是癌症能量代谢的重要特征之一。关于DDX6有多项研究阐述其可作为miRNA的下游靶点,例如,miR-518c-3p/DDX6轴作用于延缓软骨内骨化标志物的表达[19],miR-52-3p在缺氧状态下靶向DDX6可加重血管内皮细胞功能障碍[20]。长链非编码RNA(long non-coding RNA,lncRNA)是miRNA的前体,但它们经常作为竞争的内膜RNA[21]。lncRNA CASC19通过调控miR-152-3p/DDX6轴加速软骨细胞凋亡和促炎细胞因子生成,加速骨关节炎发展[22]。本研究仅阐述了DDX6-CKMT1A轴在鼻咽癌中的作用,今后还将进一步探索DDX6的上游分子,阐明与此相关的鼻咽癌发生、发展的机制,从而更好地对鼻咽癌的早前诊断、治疗及预后预测提供新思路。本研究用Western blot确定了鼻咽癌中CKMT1A和DDX6的表达,稳定性实验论证了DDX6能够破坏CKMT1A的mRNA稳定性,又通过细胞表型实验证实了CKMT1A在鼻咽癌中低表达,过表达CKMT1A可以抑制鼻咽癌细胞CNE2的迁移和增殖,以及减少细胞的克隆形成,对DDX6的抑制能够逆转敲低CKMT1A导致的恶性行为进展。上述结论通过小鼠体内实验再次得到证实。
综上所述,DDX6负调控CKMT1A从而促进鼻咽癌的进展,DDX6-CKMT1A轴对鼻咽癌诊断、治疗及预后预测具有重要意义。
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:
熊茄妍,雷伟:酝酿和设计实验,实施研究,采集数据,起草文章;
游波:酝酿和设计实验,分析和解释数据,审阅文章并指导修改;
张振新:审阅文章,行政、技术或材料支持;
谢海静:采集、分析和解释数据,统计分析;
单颖,夏天:分析和解释数据,审阅文章,统计分析,获取研究经费,指导研究;
周勇:酝酿和设计实验,审阅文章,获取研究经费,行政、技术或材料支持,指导研究。
[参考文献]
[1] YOKOTA T, HOMMA A, KIYOTA N, et al. Immunotherapy for squamous cell carcinoma of the head and neck[J]. Jpn J Clin Oncol, 2020, 50(10): 1089-1096.
[2] MO Y Z, WANG Y M, WANG Y A, et al. Circular RNA circPVT1 promotes nasopharyngeal carcinoma metastasis via the β-TrCP/c-Myc/SRSF1 positive feedback loop[J]. Mol Cancer, 2022, 21(1): 192.
[3] LU D, YUNIS J J. Cloning, expression and localization of an RNA helicase gene from a human lymphoid cell line with chromosomal breakpoint 11q23.3[J]. Nucleic Acids Res, 1992, 20(8): 1967-1972.
[4] BOURGEOIS C F, MORTREUX F, AUBOEUF D. The multiple functions of RNA helicases as drivers and regulators of gene expression[J]. Nat Rev Mol Cell Biol, 2016, 17(7): 426-438.
[5] TAJIRIKA T, TOKUMARU Y, TANIGUCHI K, et al. DEADbox protein RNA-helicase DDX6 regulates the expression of HER2 and FGFR2 at the post-transcriptional step in gastric cancer cells[J]. Int J Mol Sci, 2018, 19(7): 2005.
[6] GHASHGHAEI M, LE C T, SHAALAN H, et al. miR-148a- 3p and DDX6 functional link promotes survival of myeloid leukemia cells[J]. Blood Adv, 2023, 7(15): 3846-3861.
[7] FAN H X, LV P, MU T, et al. LncRNA n335586/miR-924/ CKMT1A axis contributes to cell migration and invasion in hepatocellular carcinoma cells[J]. Cancer Lett, 2018, 429: 89-99.
[8] YANG M J, WANG X C, YE Z H, et al. Mitochondrial creatine kinase 1 regulates the cell cycle in non-small cell lung cancer via activation of cyclin-dependent kinase 4[J]. Respir Res, 2023, 24(1): 111.
[9] SHI H D, SONG Y L, SONG Z, et al. CKMT1B is a potential prognostic biomarker and associated with immune infiltration in Lower-grade glioma[J]. PLoS One, 2021, 16(1): e0245524.
[10] AMAMOTO R, UCHIUMI T, YAGI M, et al. The expression of ubiquitous mitochondrial creatine kinase is downregulated as prostate cancer progression[J]. J Cancer, 2016, 7(1): 50-59.
[11] 李 刚. CKMT1A对人鼻咽癌HONE1细胞铂类化疗药物敏感性的影响及机制研究[J]. 中南医学科学杂志, 2019, 47(6): 576-580.
LI G. Mitochondrial creatine kinase 1A in sensitivity of nasopharyngeal carcinoma cells to platinum-based chemotherapeutics[J]. Med Sci J Cent South China, 2019, 47(6): 576-580.
[12] COLAPRICO A, SILVA T C, OLSEN C, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data[J]. Nucleic Acids Res, 2016, 44(8): e71.
[13] HAAS R C, KORENFELD C, ZHANG Z F, et al. Isolation and characterization of the gene and cDNA encoding human mitochondrial creatine kinase[J]. J Biol Chem, 1989, 264(5): 2890-2897.
[14] SU C H, TANG Y D, ZHENG C F. DExD/H-box helicases: multifunctional regulators in antiviral innate immunity[J]. Cell Mol Life Sci, 2021, 79(1): 2.
[15] HEERMA VAN VOSS M R, VAN DIEST P J, RAMAN V. Targeting RNA helicases in cancer: the translation trap[J]. Biochim Biophys Acta Rev Cancer, 2017, 1868(2): 510-520.
[16] VRIES S D, NAARMANN-DE VRIES I S, URLAUB H, et al. Identification of DEAD-box RNA helicase 6 (DDX6) as a cellular modulator of vascular endothelial growth factor expression under hypoxia[J]. J Biol Chem, 2013, 288(8): 5815-5827.
[17] AKAO Y, MATSUMOTO K, OHGUCHI K, et al. Human DEAD-box/RNA unwindase rck/p54 contributes to maintenance of cell growth by affecting cell cycle in cultured cells[J]. Int J Oncol, 2006, 29(1): 41-48.
[18] TANIGUCHI K, IWATSUKI A, SUGITO N, et al. Oncogene RNA helicase DDX6 promotes the process of c-Myc expression in gastric cancer cells[J]. Mol Carcinog, 2018, 57(5): 579-589.
[19] DU Z, YUAN J H, WU Z W, et al. Circulating exosomal circRNA_0063476 impairs expression of markers of bone growth via the miR-518c-3p/DDX6 axis in ISS[J]. Endocrinology, 2022, 163(11): bqac138.
[20] ZHAO Z Y, WU C J, HE X Y, et al. miR-152-3p aggravates vascular endothelial cell dysfunction by targeting DEAD-box helicase 6 (DDX6) under hypoxia[J]. Bioengineered, 2021, 12(1): 4899-4910.
[21] SCHWARZENBACH H, GAHAN P B. Interplay between lncRNAs and microRNAs in breast cancer[J]. Int J Mol Sci, 2023, 24(9): 8095.
[22] ZHOU C, HE T D, CHEN L J. LncRNA CASC19 accelerates chondrocytes apoptosis and proinflammatory cytokine production to exacerbate osteoarthritis development through regulating the miR-152-3p/DDX6 axis[J]. J Orthop Surg Res, 2021, 16(1): 399.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言

















#鼻咽癌# #细胞增殖# #细胞迁移# #CKMT1A# #DDX6#
32