研究重磅突破:迷幻蘑菇的致幻成分“裸盖菇素”对难治性抑郁症有奇效
2022-11-15 神经科学临床和基础 神经科学临床和基础
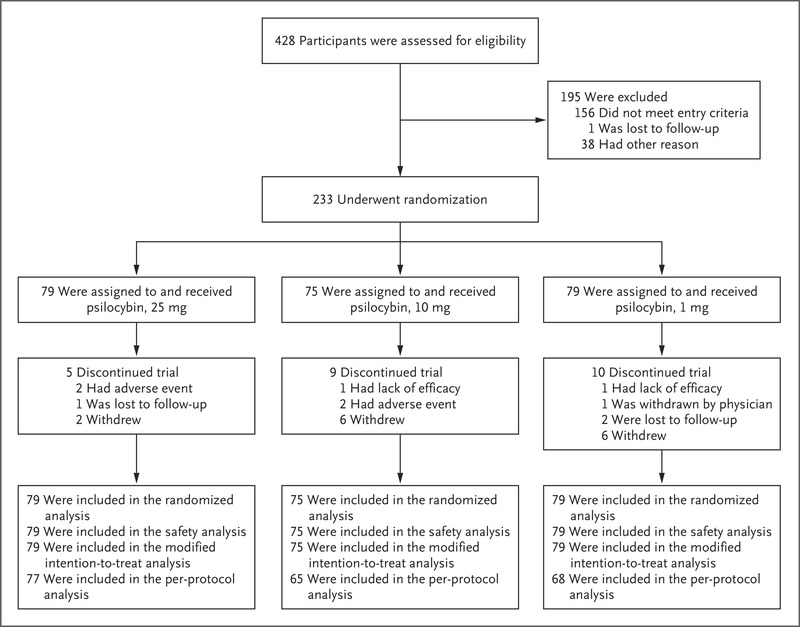
研究人员将成年难治性抑郁症患者随机分配到不同的治疗组,分别接受25 mg、10 mg或1 mg剂量(对照)的含有psilocybin的合成制剂治疗,并辅以心理支持。
 中文摘要
中文摘要
背景:Psilocybin被研究用于治疗难治性抑郁症。
方法:在这项2期双盲试验中,研究人员将成年难治性抑郁症患者随机分配到不同的治疗组,分别接受25 mg、10 mg或1 mg剂量(对照)的含有psilocybin的合成制剂治疗,并辅以心理支持。主要终点是蒙哥马利-奥斯伯格抑郁评定量表(MADRS;范围为0至60,得分越高表示抑郁越严重)总分从基线到第3周的变化。次要终点包括第3周的反应(≥MADRS总分较基线下降50%),第3周缓解(MADRS总得分≤10) ,并在12周时持续响应(在第3周和所有后续就诊时满足响应标准)。
结果:25 mg组共79名参与者,10 mg组75名参与者,1 mg组79名参与者。各组基线平均MADRS总分为32或33。从基线到第3周,评分的最小二乘均值变化为:25 mg为-12.0,10 mg为-7.9,1 mg为-5.4;25mg组和1mg组之间的差异为-6.6(95%置信区间[CI],-10.2至-2.9;P<0.001),10mg组和1-mg组之间的差别为-2.5(95%置信度,-6.2至1.2;P=0.18)。在25 mg组中,除了第12周时没有持续反应,第3周时的反应和缓解发生率总体上支持主要结果。233名参与者中有179人(77%)发生了不良反应,包括头痛、恶心和头晕。所有剂量组均出现自杀意念或行为或自伤。
结论:在这项2期试验中,患有难治性抑郁症的参与者,单剂量25 mg(而非10 mg)的psilocybin在3周内相比于1 mg剂量能显著降低抑郁评分,但有不良反应产生。需要进行更大规模、更长时间的试验,包括与现有治疗方法的比较,以确定psilocybin对该疾病的疗效和安全性。(由COMPASS Pathfinder资助;EudraCT编号,2017-003288-36;ClinicalTrials.gov编号,NCT03775200。)。
英文摘要
Background: Psilocybin is being studied for use in treatment-resistant depression.
Methods: In this phase 2 double-blind trial, we randomly assigned adults with treatment-resistant depression to receive a single dose of a proprietary, synthetic formulation of psilocybin at a dose of 25 mg, 10 mg, or 1 mg (control), along with psychological support. The primary end point was the change from baseline to week 3 in the total score on the Montgomery-Åsberg Depression Rating Scale (MADRS; range, 0 to 60, with higher scores indicating more severe depression). Secondary end points included response at week 3 (≥50% decrease from baseline in the MADRS total score), remission at week 3 (MADRS total score ≤10), and sustained response at 12 weeks (meeting response criteria at week 3 and all subsequent visits).
Results: A total of 79 participants were in the 25-mg group, 75 in the 10-mg group, and 79 in the 1-mg group. The mean MADRS total score at baseline was 32 or 33 in each group. Least-squares mean changes from baseline to week 3 in the score were -12.0 for 25 mg, -7.9 for 10 mg, and -5.4 for 1 mg; the difference between the 25-mg group and 1-mg group was -6.6 (95% confidence interval [CI], -10.2 to -2.9; P<0.001) and between the 10-mg group and 1-mg group was -2.5 (95% CI, -6.2 to 1.2; P = 0.18). In the 25-mg group, the incidences of response and remission at 3 weeks, but not sustained response at 12 weeks, were generally supportive of the primary results. Adverse events occurred in 179 of 233 participants (77%) and included headache, nausea, and dizziness. Suicidal ideation or behavior or self-injury occurred in all dose groups.
Conclusions: In this phase 2 trial involving participants with treatment-resistant depression, psilocybin at a single dose of 25 mg, but not 10 mg, reduced depression scores significantly more than a 1-mg dose over a period of 3 weeks but was associated with adverse effects. Larger and longer trials, including comparison with existing treatments, are required to determine the efficacy and safety of psilocybin for this disorder. (Funded by COMPASS Pathfinder; EudraCT number, 2017-003288-36; ClinicalTrials.gov number, NCT03775200.).
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言