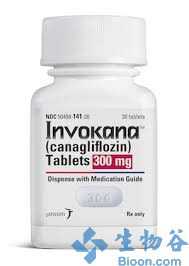
强生糖尿病新药INVOKANA获欧盟批准
2013-11-25 tomato 生物谷
强生(JNJ)旗下杨森(Janssen)宣布,糖尿病新药INVOKANA(canagliflozin)获欧盟委员会(EC)批准,用于2型糖尿病成人患者的治疗,以改善血糖控制。 今年9月,canagliflozin获得了欧洲药品管理局(EMA)人用医药产品委员会(CHMP)建议批准的积极建议。此外,canagliflozin已于今年3月获FDA批准,并于近期获得了澳大利亚的批准。 Canag
强生(JNJ)旗下杨森(Janssen)宣布,糖尿病新药INVOKANA(canagliflozin)获欧盟委员会(EC)批准,用于2型糖尿病成人患者的治疗,以改善血糖控制。
今年9月,canagliflozin获得了欧洲药品管理局(EMA)人用医药产品委员会(CHMP)建议批准的积极建议。此外,canagliflozin已于今年3月获FDA批准,并于近期获得了澳大利亚的批准。
Canagliflozin为日服一次的口服糖尿病药物,属于选择性纳葡萄糖共转运体2(sodium glucose co-transporter 2,SGLT2)抑制剂的一类新药,通过阻断肾脏对血糖的重吸收及增加尿液中血糖的排泄,来降低机体血糖水平。
与非糖尿病人群相比,2型糖尿病患者的肾脏能够重吸收大量的葡萄糖进入血液,这可能会推高血糖水平。
Canagliflozin是强生公司最有前途的药物候选者之一。该公司旗下杨森部门具有该药在北美、南美、欧洲、中东、非洲、澳大利亚、新西兰及一些亚洲国家的销售权。
英文原文:INVOKANA? (canagliflozin) Approved In The European Union For Treatment Of Adults With Type 2 Diabetes[1]
Beerse, Belgium, November 18, 2013 - Janssen-Cilag International NV (Janssen) announced today that the European Commission (EC) has approved INVOKANA? (canagliflozin) in the European Union for the treatment of adults with type 2 diabetes mellitus, to improve glycaemic control1. Canagliflozin is an oral, once-daily medication, which belongs to a new class of medications called sodium glucose co-transporter 2 (SGLT2) inhibitors.
The decision from the EC follows a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommending the approval of canagliflozin in September 2013. Canagliflozin is indicated in adults aged 18 years and older with type 2 diabetes mellitus to improve glycaemic control;
- as monotherapy, when diet and exercise alone do not provide adequate glycaemic control in patients for whom the use of metformin is considered inappropriate due to intolerance or contraindications
- or as add-on therapy with other anti-hyperglycaemic medicinal products including insulin, when these together with diet and exercise, do not provide adequate glycaemic control.
Professor Guntram Schernthaner, Department of Medicine I, Rudolfstiftung Hospital, Austria, comments “For patients with type 2 diabetes, managing blood sugar levels is a daily struggle. Nearly half of adults suffering from the disease fail to achieve or maintain adequate levels of glucose, which can lead to potentially life-threatening complications. For the growing number of patients with type 2 diabetes in Europe, the approval of a new additional treatment option is very welcome”.
The kidneys make an important contribution in controlling blood glucose levels. As glucose is filtered from the blood into the kidneys, it is reabsorbed back into the bloodstream. An important carrier responsible for this reabsorption is called sodium glucose co-transporter 2 (SGLT2). Canagliflozin selectively inhibits SGLT2, and, as a result, promotes the loss of glucose via the urine, lowering blood glucose levels in adults with type 2 diabetes. Large scale studies have demonstrated that due to the increased urinary loss of glucose, canagliflozin is also associated with reductions in both systolic blood pressure and body weight.[2]
“We are delighted that INVOKANA ? has been approved for use in Europe, as it presents another innovative treatment option for patients with type 2 diabetes, helping them manage this progressive disease. The approval of canagliflozin also reinforces Janssen’s commitment to addressing the unmet needs in the treatment and management of type 2 diabetes,” comments Jane Griffiths, Company Group Chairman, Janssen Europe, Middle-East, Africa.
The approval by the EC was based on the analysis of data from two Phase III trials The approval by the EC was based on the analysis of data from two Phase III trials The approval by the EC was based on the analysis of data from two Phase III trials The approval by the EC was based on a comprehensive global Phase 3 clinical trial programme, which enrolled 10,285 patients in nine studies[2-11], and is one of the largest late-stage development programmes for an investigational pharmacological product for the treatment of type 2 diabetes submitted to health authorities to date.
Three studies have compared canagliflozin to current standard treatments[3-5]; two of which compared canagliflozin to sitagliptin[3,4] and the other to glimepiride.[5] The Phase 3 programme also included three large studies in special populations[6-8]: patients over 55 with type 2 diabetes[6], patients with type 2 diabetes who had moderate renal impairment7, and patients with type 2 diabetes who were considered to be at high risk for cardiovascular disease.[8]
Results from the programme showed that both the 100 mg and the 300 mg doses of canagliflozin improved glycaemic control, compared to baseline. A secondary study endpoint showed that there was body weight reduction in the canagliflozin groups compared to those on placebo. Phase 3 results showed that canagliflozin was generally well-tolerated. Adverse drug reactions due to SGLT2 inhibition and also associated with canagliflozin included genital mycotic infections, urinary tract infections (UTIs), osmotic diuresis (such as urinary frequency, thirst or constipation), reduced intravascular volume (such as postural dizziness). Canagliflozin was also associated with a low incidence of rash or urticaria. The frequency of hypoglycaemia was low when canagliflozin was used as a monotherapy, or as an add-on to metformin.[2]
Canagliflozin was first approved by the U.S. Food and Drug Administration (FDA) in March 2013 and recently also in Australia.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言











#ANA#
63
#INVOKANA#
64
#强生#
84
#欧盟批准#
75