癌症预防的临床科研如何做?美国在研的134项宫颈癌基金给你答案(2024)
2024-11-04 Hanson临床科研 Hanson临床科研 发表于上海
我们仅对美国国立卫生研究院(NIH)资助的在研宫颈癌相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
犹记得儿子11岁年度体检时,医生问是否愿意接种HPV疫苗;也是免费接种,但男孩自己决定是否接种,医生有推荐、但不做明确的建议。
我当时和儿子稍一解释后,毫不犹豫地选择了接种。
因为男性HPV疫苗接种,在阻断HPV感染中同样起到重要作用;另外,HPV疫苗非常安全。
当然,在美国,女孩接种HPV疫苗早已经成为常规的疫苗接种。
HPV疫苗的接种意义
宫颈癌(Cervical Cancer)是起源于子宫颈的一种恶性肿瘤,是世界上第四常见的女性癌症。根据世界卫生组织的数据,每年有超过五十万女性被诊断患有宫颈癌,其中约有50%的患者会死于这种疾病。HPV是宫颈癌的主要原因。
2016年Lancet PH文章建模指出,如果全世界HPV疫苗接种率达到80%,将彻底消除HPV 16/18,从而基本杜绝宫颈癌等疾病
尽管近年来在宫颈癌的诊断和治疗方面取得了显著进展,但仍存在一些重要而未解决的临床问题:
-
早期筛查和诊断:尽管有Pap涂片和HPV DNA检测等有效的筛查工具,但在全球范围内,特别是低资源国家,筛查覆盖率仍然不足。改进这些地区的筛查和诊断设施是当务之急。
-
疫苗接种率:虽然HPV疫苗已被证明可以预防大部分宫颈癌,但在很多国家,特别是发展中国家,疫苗的接种率仍然较低。
-
治疗方法的改进:对于早期宫颈癌,手术和放射治疗非常有效,但对于晚期宫颈癌,治疗效果仍有限。研究新的治疗方法,包括靶向疗法和免疫疗法,是当前的研究热点。
-
跟踪和管理HPV感染:如何有效地跟踪和管理持续的HPV感染以预防宫颈癌的发展是一个重要问题。现有的管理策略需要根据不同地区的具体情况进行调整。
总体来说,尽管在预防、筛查和治疗宫颈癌方面取得了显著进展,但全球范围内仍需加强这些努力,尤其是在资源有限的地区。提高疫苗接种率、改进筛查覆盖和发展新的治疗方法是未来工作的关键方向。
我们仅对美国国立卫生研究院(NIH)资助的在研宫颈癌相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Cervical Cancer”为检索词、在题目中进行检索,美国NIH针对宫颈癌的在研有134项。
这些可以对于申请癌症预防的医生科学家,会有很多启发。
一,谁获得了这些研究?
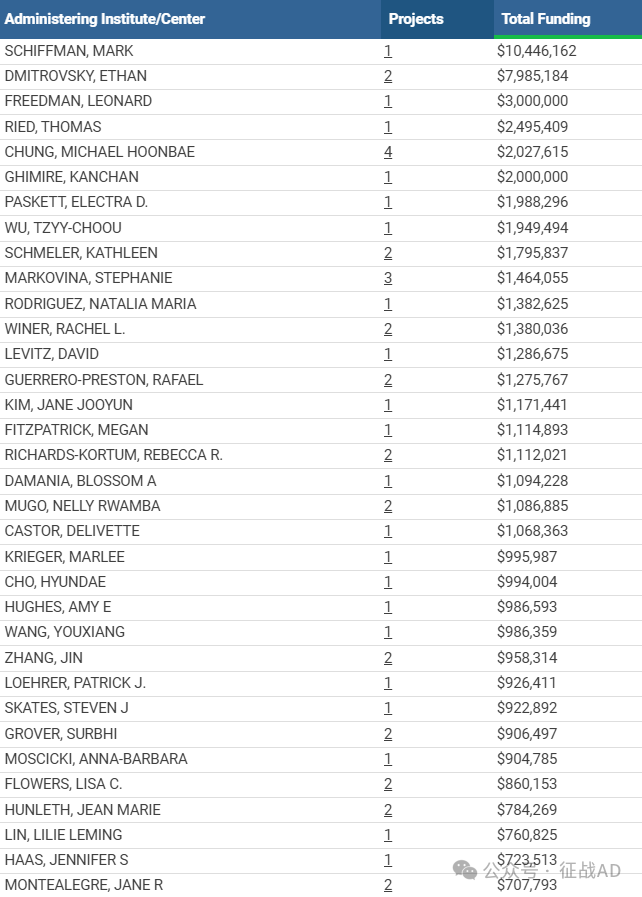
1,在研宫颈癌基金最多的PI
-
DIVISION OF CANCER EPIDEMIOLOGY AND GENETICS 的 SCHIFFMAN, MARK
-
LEIDOS BIOMEDICAL RESEARCH, INC. 的 DMITROVSKY, ETHAN
-
LEIDOS BIOMEDICAL RESEARCH, INC. 的 FREEDMAN, LEONARD
-
DIVISION OF BASIC SCIENCES - NCI 的 RIED, THOMAS
-
EMORY UNIVERSITY 的 CHUNG, MICHAEL HOONBAE
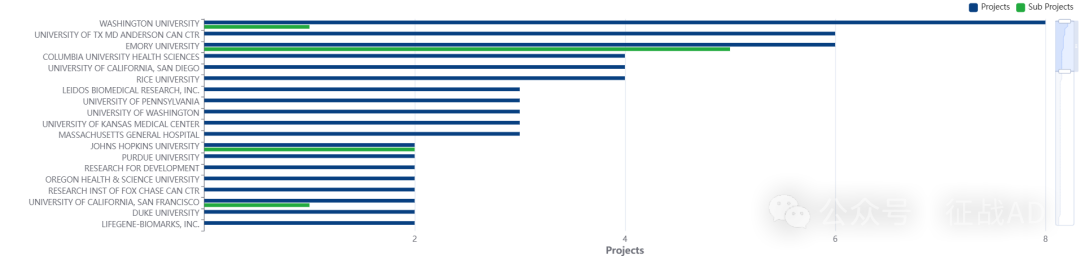
2,宫颈癌基金最多的研究机构
-
LEIDOS 生物医学研究公司
-
癌症流行病学和遗传学部门
-
华盛顿大学
-
德克萨斯大学 MD 安德森癌症中心
-
埃默里大学等
二,宫颈癌研究热点是什么?
宫颈癌研究领域总览(根据关键词)
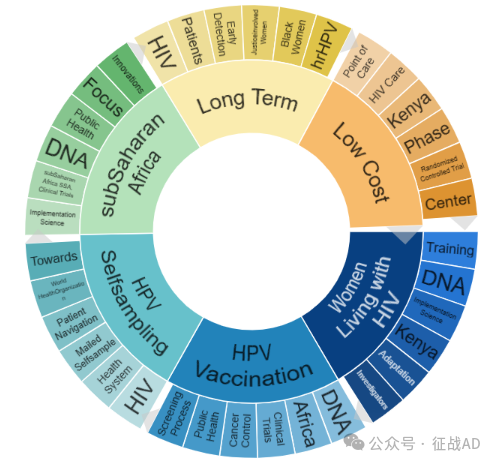
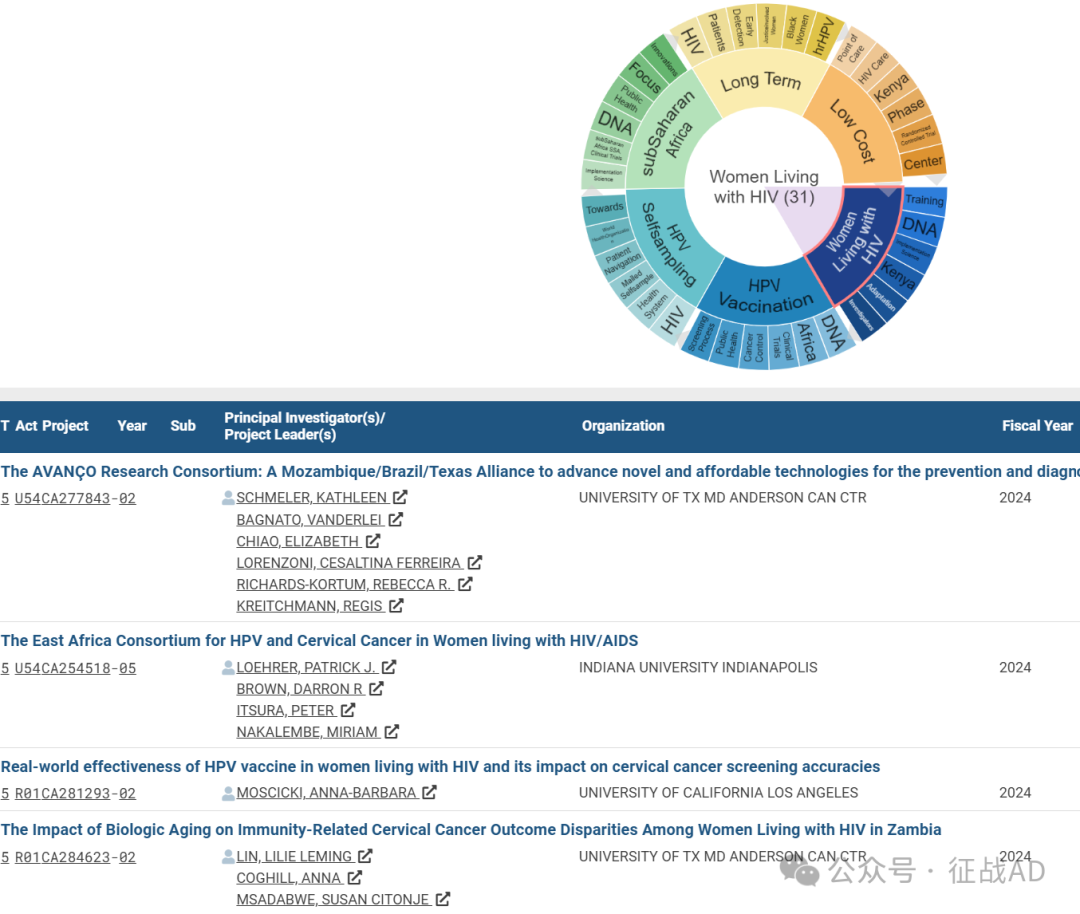
A,关于感染HIV的女性(Women Living with HIV)的研究项目最多
有 31 项在研基金涉及到了感染HIV的女性,关注最多的方面包括培训(Training)、DNA、执行科学(Implementation Science)、肯尼亚(Kenya)、适应性(Adaptation)、调查员(Investigatiors)等方面研究。
B,HPV 疫苗接种(HPV Vaccination)的研究
有 26 项研究涉及到HPV 疫苗接种,研究领域主要涉及DNA、非洲(Africa)、临床试验(Clinical Trials)、癌症控制(Cancer Control)、公共卫生(Public Health)、筛查过程(Screening Process)等方面研究。
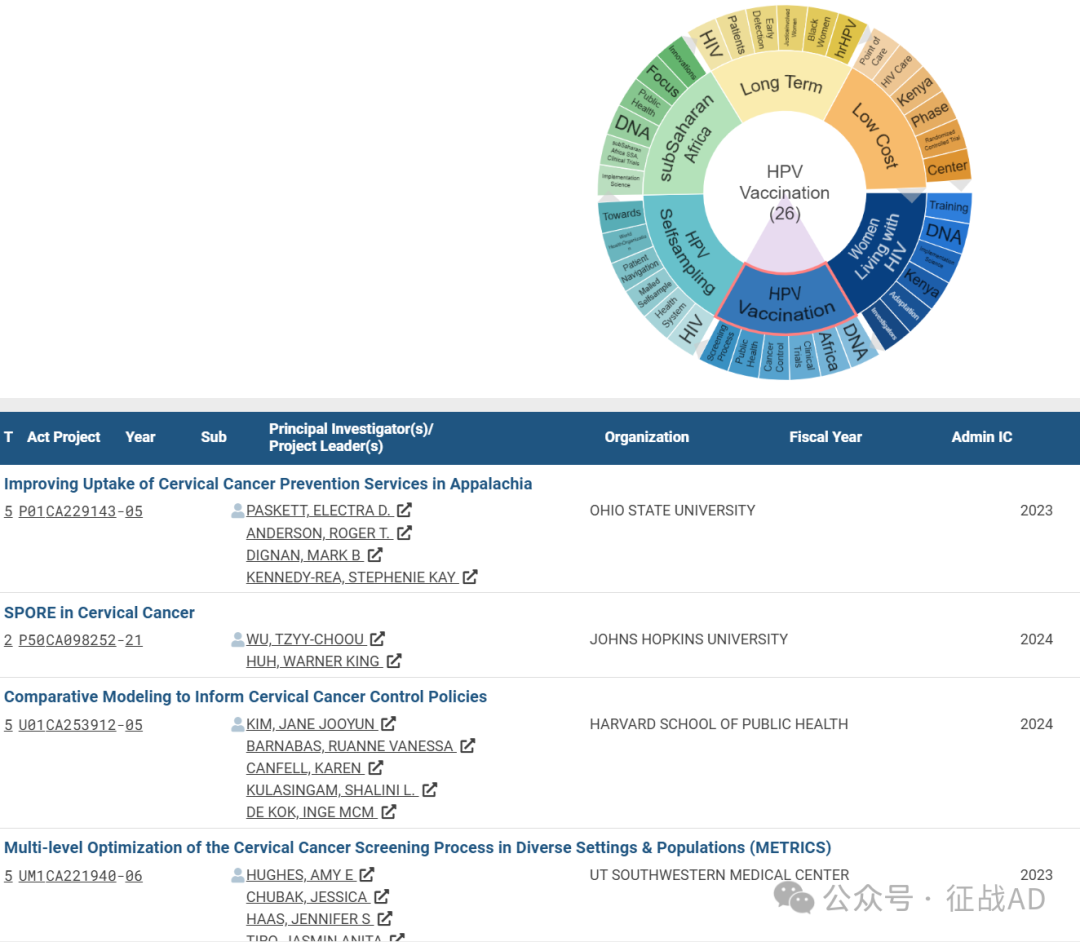
C,HPV 自我采样(HPV Selfsampling)
有 23 项研究涉及到HPV 自我采样,涉及的关键词包括如HIV、保健系统(Health System)、邮寄自采样(Mailed Selfsample)、患者导航(Patient Navigation)、WHO等。
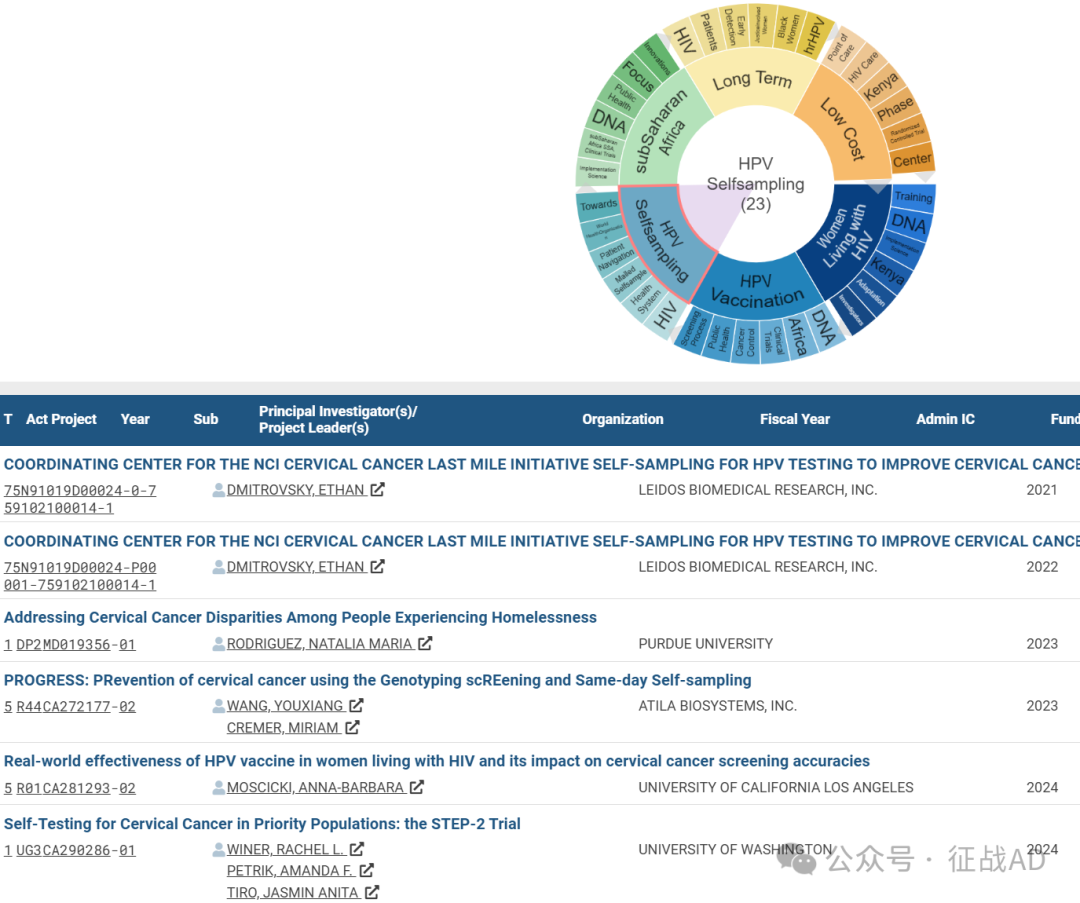
其他宫颈癌研究大的方向也包括撒哈拉以南非洲(subSaharan Africa)、长期(Long Term)、低成本(Low Cost)等。
三,借鉴与突破
我们也分享宫颈癌领域的几项课题摘要,希望对同仁们有所启发。
A,COORDINATING CENTER FOR THE NCI CERVICAL CANCER LAST MILE INITIATIVE SELF-SAMPLING FOR HPV TESTING TO IMPROVE CERVICAL CANCER PREVENTI
An alternative screening approach under evaluation to overcome these barriers is self-collection of samples (‘self-sampling’) by women themselves and sending the sample for HPV testing. This approach offers several benefits including ease of collection at a time/place of women’s choice without a need for a clinic appointment or a speculum examination. This intervention has significant potential to expand cervical cancer screening to never screened or under-screened women and address a pressing public health concern of lack of access to screening as a health disparity.
The National Cancer Institute (NCI) has developed the Cervical Cancer ‘Last Mile’ Initiative as a public private partnership between several stakeholders (including federal agencies, industry partners, and professional societies/clinical practice guidelines organizations) to validate self-sampling-based HPV testing as a comparable (non-inferior) alternative to provider-collected cervical specimen for HPV testing in cervical cancer screening, and facilitate regulatory approvals and broader adoption of this approach in real world practice settings.
Towards this goal, the NCI will support a nationwide, multicentric screening trial, the Self-sampling for HPV testing to Improve Cervical Cancer Prevention (SHIP)” Trial (‘SHIP Trial’) in diverse delivery settings. Pending discussions with the US Food and Drug Administration (FDA) and the industry partners, the SHIP Trial may be designed as a pre-approval trial or a post-approval trial, dependent on the approved regulatory pathways.
B, Improving Uptake of Cervical Cancer Prevention Services in Appalachia
The goal of this Program Project is to address the burden of cervical cancer incidence and mortality in Appalachia through the delivery of a clinic-based integrated prevention program that focuses on the major causes of cervical cancer (tobacco smoking, Human Papillomavirus (HPV) infection, and lack of cervical cancer screening) designed to target individual, social and community, health system and broader contextual-level barriers related to the burden of cervical cancer. Building upon our long history of collaborative research and community partnerships, the Program will test the effectiveness of health system-based interventions focused on tobacco use, HPV vaccination and cervical cancer screening (Pap test and/or self-testing with follow-up of positive tests), as part of an integrated clinic-based cervical cancer prevention program. The multi-level interventions (directed to three levels of influence – clinic, provider and patient) will be offered to eligible female patients and age-eligible children and young adults in four Appalachian states (Ohio, Kentucky, West Virginia and Virginia). Our research process is guided by a socio-ecological model based on the Social Determinants of Health, the Proctor Model for Implementation Science and a Community-Based Participatory Research (CBPR) framework.
The aims of this Program Project are to: 1) Test the effectiveness of an integrated cervical cancer prevention program, consisting of three established interventions, designed to address three causes of cervical cancer in a region with one of the highest cervical cancer incidence and mortality rates in the United States; and 2) Evaluate the impact of the cervical cancer prevention program, including implementation, and acceptability, with attention to both short- and long-term impact and sustainability at the clinics.
Four cores – Intervention and Consortium, Survey and Data Collection, Biostatistics and Evaluation, and Administrative – will facilitate the smooth and integrated operation of all projects.
Integration and interaction of the projects in this Program is evident in several ways: 1) all projects focus on one health disparity; 2) participants will be recruited from the same health systems; 3) a core set of measures is being used by all projects; 4) all projects include transdisciplinary teams; 5) all projects build upon and extend findings from our long history of collaborative research and community partnerships; 6) all projects focus on multi-level assessment and/or interventions using the Warnecke model as a framework and utilize the Proctor et al. Implementation Science Framework; 7) all projects involve interaction with the community in some way, thus enhancing the CBPR nature of the Program; and 8) evaluation will assess implementation, service and client outcomes at the project and overall Program levels including: cost, satisfaction, effectiveness, sustainability, and safety, to name a few outcomes.
If successful, this Program Project will provide evidence for a novel and innovative approach to address disparities in underserved communities with plans for sustainability and dissemination, as well as cost-effectiveness data.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言
















#宫颈癌# #美国国立卫生研究院#
39