Lancet Neurol—Tau蛋白生物标志物研究的现状及未来
2022-12-05 神经科学临床和基础 神经科学临床和基础 发表于安徽省
Tau聚集体的沉积是阿尔茨海默病的病理特征,在空间和时间上与神经变性的出现和临床症状的表现密切相关。
 英文摘要
英文摘要
Background: Deposition of tau aggregates is a pathological hallmark of Alzheimer's disease that is closely linked both spatially and temporally to emergence of neurodegeneration and manifestation of clinical symptoms. There is an urgent need for accurate PET, CSF, and plasma biomarkers of tau pathology to improve the diagnostic process in clinical practice and the selection of participants and monitoring of treatment effects in trials.
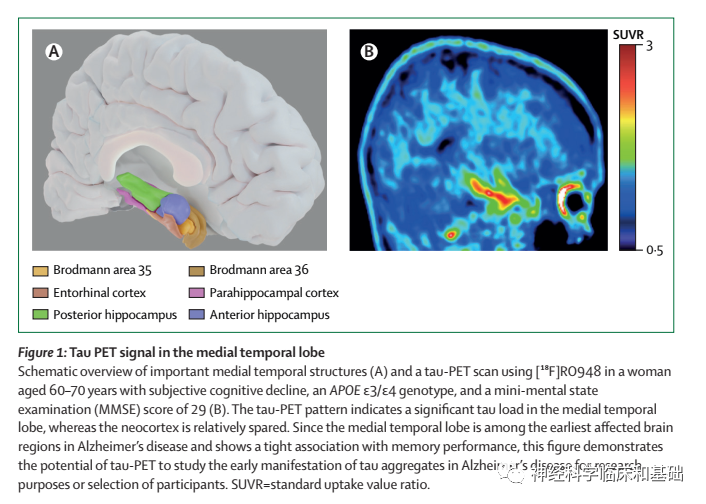
Recent developments: Innovative second-generation tau-PET tracers with high affinity and selectivity to tau pathology in Alzheimer's disease have enabled detection of tau pathology in medial temporal lobe subregions that are affected in the earliest disease stages. Furthermore, novel but common tau spreading subtypes have been discovered using tau-PET, suggesting much greater interinpidual differences in the distribution of tau pathology across the brain than previously assumed. In the CSF biomarker field,novel phosphorylated tau (p-tau) assays have been introduced that better reflect tau tangle load than established CSF biomarkers of tau pathology. The advent of cost-effective and accessible blood-based biomarkers for tau pathophysiology (ie, p-tau181, p-tau217, and p-tau231) might transform the Alzheimer's disease field, as these biomarkers correlate with post-mortem Alzheimer's disease pathology, differentiate Alzheimer's disease from other types of dementia, and predict future progression from normal cognition and mild cognitive impairment to Alzheimer's disease. In controlled investigational settings, improvements in tau-PET and biofluid p-tau markers have led to earlier disease detection, more accurate diagnostic methods, and refinement of prognosis. The anti-tau therapy landscape is rapidly evolving, with multiple ongoing phase 1 and 2 trials of post-translational modification of tau, tau immunotherapy, tau aggregation inhibitors, and targeting production of tau and reduction of intracellular tau levels. Neuroimaging and biofluid tau markers hold potential for optimising such clinical trials by augmenting participant selection, providing evidence of target engagement, and monitoring treatment efficacy. WHERE NEXT?: Major challenges to overcome are the high cost of tau-PET, partial sensitivity to detect early-stage Alzheimer's disease pathology, and off-target tracer binding. Prospective validation studies of biofluid p-tau markers are needed, and assay-related preanalytical and analytical factors need further refinement. Future studies should focus on demonstrating the diagnostic and prognostic accuracy of tau biomarkers-blood-based markers in particular-in non-tertiary settings, such as primary care, which is characterised by a perse population with medical comorbidities. Large-scale head-to-head studies are needed across different stages of Alzheimer's disease to determine which tau biomarker is optimal in various clinical scenarios, such as early diagnosis, differential diagnosis, and prognosis, and for aspects of clinical trial design, such as proving target engagement, optimising participant selection, and refining monitoring of treatment effects.
 中文摘要
中文摘要
背景:Tau聚集体的沉积是阿尔茨海默病的病理特征,在空间和时间上与神经变性的出现和临床症状的表现密切相关。目前临床上迫切需要精确的PET、CSF和血浆tau病理学生物标记物,以改进临床实践中的诊断过程,以及对试验中参与者进行选择和治疗效果的监测。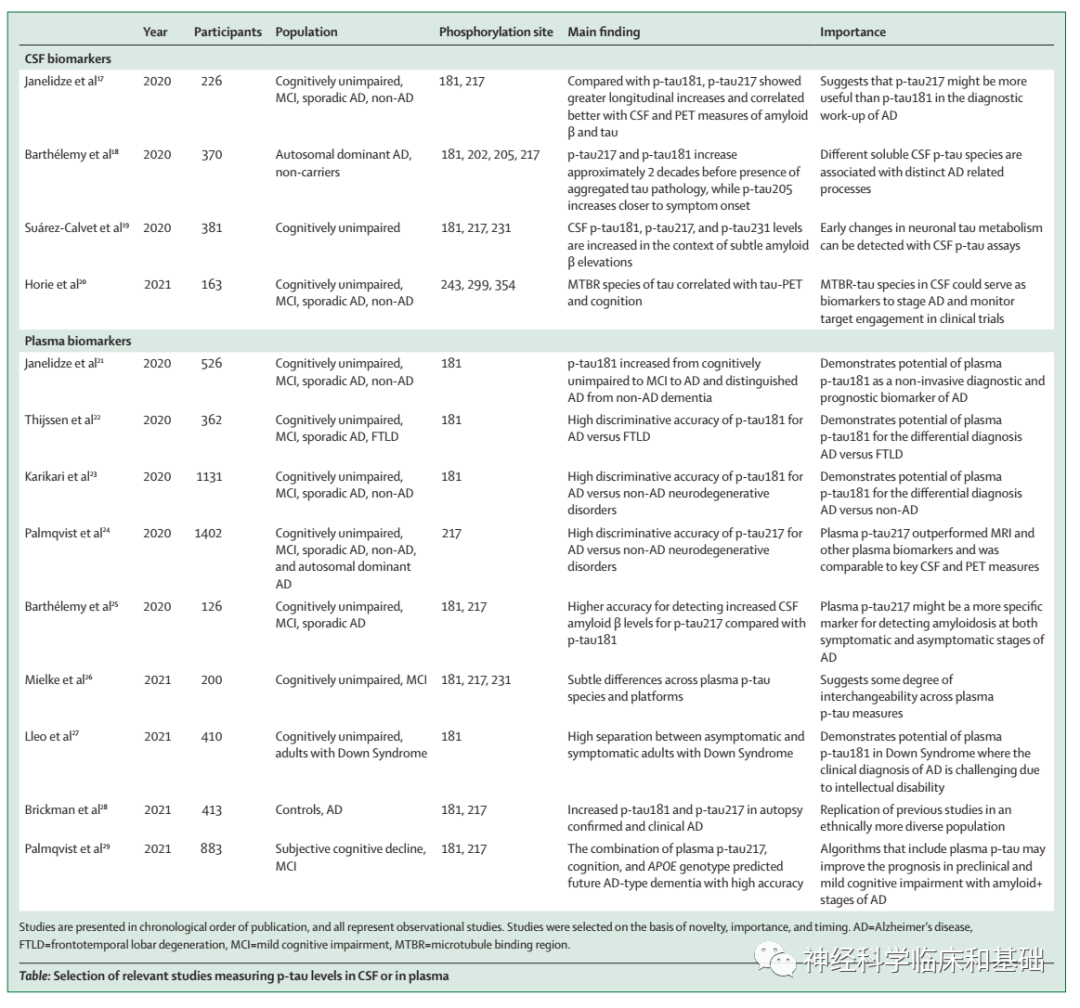 最近的发展:创新的第二代tau-PET示踪剂对阿尔茨海默病中的tau病理具有高亲和力和高选择性,能够检测到在疾病早期受影响的内侧颞叶区的tau蛋白病变。此外,使用tau-PET发现了新的且常见的tau病理播散亚型,这表明tau病理在大脑中的分布在个体间的差异要比先前假设的大得多。在脑脊液生物标记物领域,科学家已经引入了新的磷酸化Tau(p-tau)分析方法,比已建立的Tau病理学脑脊液生物学标记物更能反映tau缠结的病理负荷。针对Tau病理生理学(即p-tau181、p-tau217和p-tau231)的具有成本效益且易于获取的血液生物标记物的出现可能会改变阿尔茨海默病领域,因为这些生物标记物与死后阿尔茨海默病病理学相关,将阿尔茨海默病与其他类型的痴呆区分开来,并预测从正常认知和轻度认知障碍到阿尔茨海默病的转变。在受控试验环境中,tau-PET和生物体液p-tau标记物的改进促进了疾病的早期检测、准确诊断和精确预后。抗Tau疗法的前景正在迅速发展,目前正在进行多个关于Tau翻译后修饰、Tau免疫疗法、Tau聚集抑制剂、靶向生成Tau和降低细胞内Tau水平的第1和第2阶段试验。神经影像学和生物体液tau标记物通过促进参与者选择、提供靶点和监测治疗效果,具有优化此类临床试验的潜力。
最近的发展:创新的第二代tau-PET示踪剂对阿尔茨海默病中的tau病理具有高亲和力和高选择性,能够检测到在疾病早期受影响的内侧颞叶区的tau蛋白病变。此外,使用tau-PET发现了新的且常见的tau病理播散亚型,这表明tau病理在大脑中的分布在个体间的差异要比先前假设的大得多。在脑脊液生物标记物领域,科学家已经引入了新的磷酸化Tau(p-tau)分析方法,比已建立的Tau病理学脑脊液生物学标记物更能反映tau缠结的病理负荷。针对Tau病理生理学(即p-tau181、p-tau217和p-tau231)的具有成本效益且易于获取的血液生物标记物的出现可能会改变阿尔茨海默病领域,因为这些生物标记物与死后阿尔茨海默病病理学相关,将阿尔茨海默病与其他类型的痴呆区分开来,并预测从正常认知和轻度认知障碍到阿尔茨海默病的转变。在受控试验环境中,tau-PET和生物体液p-tau标记物的改进促进了疾病的早期检测、准确诊断和精确预后。抗Tau疗法的前景正在迅速发展,目前正在进行多个关于Tau翻译后修饰、Tau免疫疗法、Tau聚集抑制剂、靶向生成Tau和降低细胞内Tau水平的第1和第2阶段试验。神经影像学和生物体液tau标记物通过促进参与者选择、提供靶点和监测治疗效果,具有优化此类临床试验的潜力。
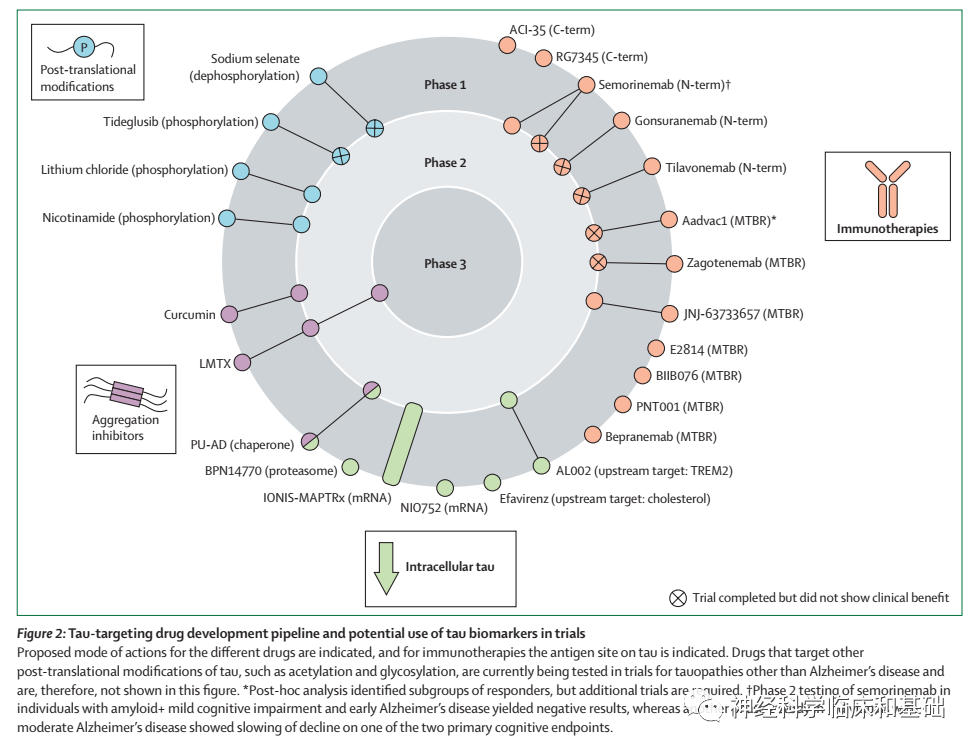 Tau蛋白研究的未来:在这个领域,要克服的主要挑战是tau-PET的高成本、检测早期阿尔茨海默病病理学的部分敏感性以及非靶向示踪剂结合。需要对生物体液p-tau标记进行前瞻性验证研究,需要进一步完善分析前和分析中的混杂因素。未来的研究应侧重于证明tau生物标记物中基于血液的标记物的诊断和预后准确性,尤其是在非三级诊疗环境中,如初级保健,其特点是具有不同的医学共病人群。未来需要在阿尔茨海默病的不同阶段进行大规模的头对头研究,以确定哪种tau生物标记物在哪个临床阶段中是最佳的,例如早期诊断、鉴别诊断和预后。此外,也需要确定其在临床试验设计方面的价值,例如证明靶点参与度、优化参与者选择和完善治疗效果监测。
Tau蛋白研究的未来:在这个领域,要克服的主要挑战是tau-PET的高成本、检测早期阿尔茨海默病病理学的部分敏感性以及非靶向示踪剂结合。需要对生物体液p-tau标记进行前瞻性验证研究,需要进一步完善分析前和分析中的混杂因素。未来的研究应侧重于证明tau生物标记物中基于血液的标记物的诊断和预后准确性,尤其是在非三级诊疗环境中,如初级保健,其特点是具有不同的医学共病人群。未来需要在阿尔茨海默病的不同阶段进行大规模的头对头研究,以确定哪种tau生物标记物在哪个临床阶段中是最佳的,例如早期诊断、鉴别诊断和预后。此外,也需要确定其在临床试验设计方面的价值,例如证明靶点参与度、优化参与者选择和完善治疗效果监测。
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言