Sci Transl Med:两种药物结合可有效阻止肝癌的发生
2012-05-24 T.Shen 生物谷
近日,来自Bellvitge生物医药研究院的研究者研究发现,两种mTOR蛋白抑制剂结合之后会阻止原发性肝癌的发展并且可以破坏肿瘤细胞,相关研究成果刊登在了近日的国际杂志Science Translational Medicine上。 原发性肝癌或肝细胞癌是世界上第五大常见的癌症,因其强烈的攻击性,是世界上第三大致死性疾病。在世界范围内,该疾病影响着50万人的健康,每三个患者中有两个和慢性酒精中毒

近日,来自Bellvitge生物医药研究院的研究者研究发现,两种mTOR蛋白抑制剂结合之后会阻止原发性肝癌的发展并且可以破坏肿瘤细胞,相关研究成果刊登在了近日的国际杂志Science Translational Medicine上。
原发性肝癌或肝细胞癌是世界上第五大常见的癌症,因其强烈的攻击性,是世界上第三大致死性疾病。在世界范围内,该疾病影响着50万人的健康,每三个患者中有两个和慢性酒精中毒、接触有毒制剂、乙肝病毒和丙肝病毒有关;其余的患者和非酒精性的脂肪肝(肥胖疾病)有关系。

候选药物
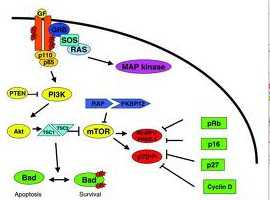
目前,抗癌药物索拉非尼在病人治疗上表现出了较好的效果,但是随着时间延续,药物的效用却在慢慢降低。因此,寻找新的治疗方法迫在眉睫。在许多潜在的药物中,mTOR信号途径的抑制剂表现出更大的治疗效果和价值。mTOR途径在肝细胞癌细胞中可以被高度激活。
研究者在小鼠体内比较了两种mTOR抑制剂的效应,第一种抑制剂是雷帕霉素的衍生物,成为依维莫司(RAD001),已经作为免疫抑制剂治疗特殊的癌症;第二种是一种新一代的药物,称为BEZ235,可以抑制mTOR途径。
在延吉中,研究者意外地发现,相比单一使用一种药物进行治疗,将以上两种药物结合以后来治疗,表现出更好的治疗效果。同时服用BEZ235和RAD001以后,可以明显抑制肿瘤细胞的生长和发育,可以促使肿瘤细胞自我毁灭。
由诺华公司提供支持,而且基于临床研究的数据,目前美国已经开始评估两种药物联合使用的具体效用。研究者Sara Kozma表示,因为雷帕霉素已经批准用于治疗其它的疾病,如果结合上BEZ235,将会是一种更为有用的治疗药物,而且临床数据也显示出两种药物结合后的较好的治疗效果。(生物谷:T.Shen编译)

doi:10.1126/scitranslmed.3003923
PMC:
PMID:
mTOR Inhibitors Synergize on Regression, Reversal of Gene Expression, and Autophagy in Hepatocellular Carcinoma
Hala Elnakat Thomas1,2, Carol A. Mercer2, Larissa S. Carnevalli1,*, Jongsun Park1,2,†, Jesper B. Andersen3, Elizabeth A. Conner3, Kazuhiro Tanaka4, Tomoo Matsutani4, Akio Iwanami4, Bruce J. Aronow5, Liu Manway6, S. Michel Maira7, Snorri S. Thorgeirsson3, Paul S. Mischel4, George Thomas1,2,8 and Sara C. Kozma1,2,8,‡
Hepatocellular carcinoma (HCC) affects more than half a million people worldwide and is the third most common cause of cancer deaths. Because mammalian Target of Rapamycin (mTOR)-signaling is upregulated in 50% of HCCs, we compared the effects of the FDA-approved mTOR-allosteric inhibitor, RAD001, with a new generation PI3K/mTOR ATP-site competitive inhibitor, BEZ235. Unexpectedly, the two drugs acted synergistically in inhibiting the proliferation of cultured HCC cells. The synergistic effect closely paralleled 4E-BP1 dephosphorylation, which is implicated in the suppression of tumor cell proliferation. In a mouse model approximating human HCC, the drugs in combination, but not singly, induced a dramatic regression in tumor burden. However in the tumor, BEZ235 alone was as effective as the combination in inhibiting 4E-BP1 phosphorylation, which suggest additional target(s) may also be involved. Microarray analyses revealed a large number of genes that reverted to normal liver tissue expression in mice treated with both drugs, but not either drug alone. These analyses also revealed the down regulation of autophagy genes in tumors compared to normal liver. Moreover, in HCC patients, altered expression of autophagy genes was associated with poor prognosis. Consistent with these findings, the drug combination had a profound effect on UNC51-like kinase 1 (ULK1) dephosphorylation and autophagy in culture, independent of 4E-BP1, and in parallel induced tumor mitophagy, a tumor-suppressor process in liver. These observations have led to an investigator-initiated Phase 1B-2 dose escalation trial with RAD001 combined with BEZ235 in patients with HCC and other advanced solid tumors.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言













#TRA#
70
#Transl#
70
#Med#
65