肾移植,仍有这些问题亟待解决!美国42项在研基金,暴露了这些热点;两个案例尤有启发(2024)
2024-09-26 Hanson临床科研 Hanson临床科研 发表于上海
我们仅对美国国立卫生研究院(NIH)资助的在研肾移植相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
肾移植(Kidney Transplant)是一种治疗严重肾功能衰竭或终末期肾病(ESRD)的外科手术方法。在此手术中,一颗健康的肾脏从供体转移到患者体内,以取代患者失功能的肾脏。肾移植通常被视为比长期透析更优的治疗选择,因为它可以提供更高质量的生活和更佳的生存率。
【未解决的临床问题】
-
供体短缺:需求远远超过供应,许多患者可能需要等待多年才能进行肾移植。
-
排斥反应:即便是在良好匹配和管理下,长期的免疫抑制治疗也可能导致严重的副作用,如感染和肿瘤风险增加。
-
药物副作用和管理:免疫抑制药物虽然可以防止排斥,但长期使用可能对心血管健康等产生负面影响。
-
移植后监测和治疗:需要持续监控患者的药物水平、肾功能和其他健康指标,确保移植肾的健康和功能。
-
再次移植的复杂性:对于第一次移植失败的患者,再次移植涉及更多的复杂性和风险。
肾移植领域的未来研究重点包括改善免疫抑制策略,减少慢性排斥和延长移植肾存活时间,以及开发替代供体来源如干细胞和生物工程肾脏。此外,提高供体的可用性和质量,优化患者匹配和选择流程也是持续关注的重点。
我们仅对美国国立卫生研究院(NIH)资助的在研肾移植相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Kidney Transplant”为检索词、在题目中进行检索,美国NIH针对肾移植的在研有42项。
一,谁获得了这些研究?
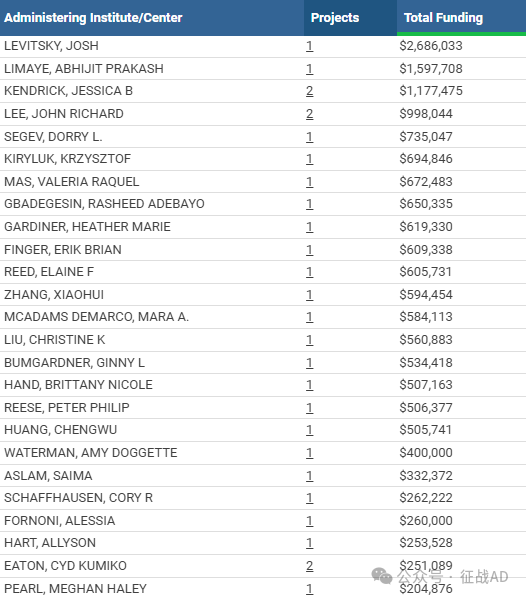
1,在研肾移植基金最多的PI
-
NORTHWESTERN UNIVERSITY AT CHICAGO 的 LEVITSKY, JOSH
-
UNIVERSITY OF WASHINGTON 的 LIMAYE, ABHIJIT PRAKASH
-
UNIVERSITY OF COLORADO DENVER 的 KENDRICK, JESSICA B
-
WEILL MEDICAL COLL OF CORNELL UNIV 的 LEE, JOHN RICHARD
-
NEW YORK UNIVERSITY SCHOOL OF MEDICINE 的 SEGEV, DORRY L.
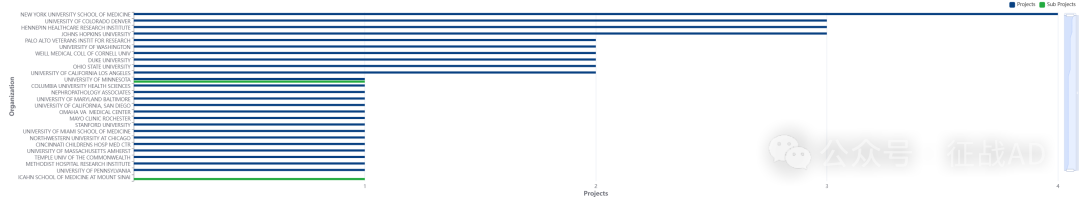
2,肾移植课题最多的研究机构
-
纽约大学医学院
-
科罗拉多大学丹佛分校
-
亨内平医疗研究所
-
约翰霍普金斯大学
-
帕洛阿尔托退伍军人研究所等。
二,肾移植研究热点是什么?
肾移植研究领域总览(根据关键词)
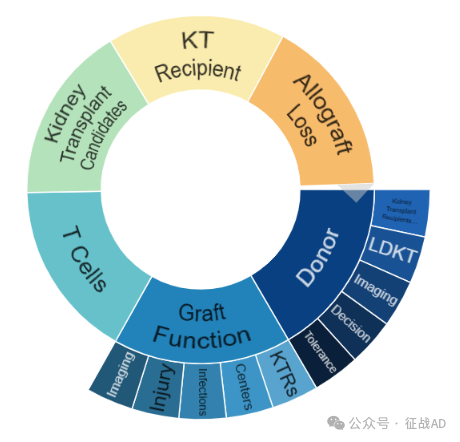
A,关于捐助者(Donor)的研究项目最多
有 13 项在研基金涉及到了捐助者,关注最多的方面包括肾移植患者(Kidney Transplant Recipients)、LDKT、影像(Imaging)、决策(Decision)、耐受性(Tolerance)等方面研究。

B,移植物功能(Graft Function)的研究
有 11 项研究涉及到移植物功能,研究领域主要涉及关键技术参数(KTRs)、中心(Centers)、感染(Infections)、损伤(Injury)、影像(Imaging)等方面研究。

其他肾移植研究大的方向也包括T细胞(T Cell)、肾移植候选人(Kidney Transplant Candidates)、肾移植接受者(KT Recipient)、同种异体移植失败(Allograft Loss)等。
三,借鉴与突破
我们也分享在肾移植领域的几项课题摘要,希望对同仁们有所启发。
A,Non-APOL1 genetic factors and kidney transplant outcomes
This is an ancillary study to the prospective APOLLO (APOL1 Long-term Kidney Transplantation Outcomes) cohort of 2,800 kidney transplant donor-recipient pairs. The parent study aims to test the impact of APOL1 risk genotypes on kidney transplantation outcomes. Here, we propose to test the role of additional genetic factors other than APOL1 in determining allograft outcomes. Accordingly, we propose to expand the scope of the APOLLO study to generate high-quality genome-wide SNP and exome sequence data for all 2,800 donor- recipient pairs enrolled by the network. Our proposal addresses the existing disparities in research and clinical care, since African-ancestry patients with end stage kidney disease are currently under-represented in genetic studies and have worse transplantation outcomes compared to non-African ancestry patients.
Our overarching hypothesis is that there are multiple additional genetic factors in this population that convey the risk of allograft loss independently of APOL1. Our recent work clearly demonstrates that polygenic background and APOL1 risk genotypes have additive effects on the risk of kidney disease in individuals of African ancestry. Our newly proposed genome-wide polygenic score (GPS) combining polygenic and APOL1 risk provided substantially improved prediction of kidney disease. There is now an urgent need to test whether combining donor polygenic and APOL1 risk improves the prediction of allograft outcomes. Additionally, our proposed generation of genome-wide genetic data will facilitate unbiased scans for specific APOL1 modifiers with an effect on graft survival. Lastly, the APOLLO study provides us with a unique opportunity to perform genetic compatibility scans in full donor-recipient pairs. We aim to test our original “genomic collision” hypothesis at the LIMS1 locus under which the recipients carrying gene-disrupting variants are at a higher risk of rejection when exposed to a graft expressing intact gene products. We will then expand this hypothesis to various types of genetic variation genome-wide, including gene-disrupting copy number variants, predicted loss-of-function variants, and even missense variants. Any positive findings from our discovery studies will be tested for validation in the ancestrally diverse international cohorts of the iGeneTRAiN consortium.
B, Microbial Biomarkers for Diagnosing and Predicting Infections in Kidney Transplant Recipients
The long term goal of this study is to develop comprehensive microbial biomarkers in the stool, blood, and the urine for diagnosing and predicting infections in kidney transplant recipients.
Our objectives are: 1) Develop a fecal SCFA assay that predicts the risk for infections 2) Develop blood and urine cfDNA assays that can comprehensively diagnose infections as well as predict the risk for infections. These objectives are inspired by observations from several recent pilot studies: 1) We have found that the fecal abundance of short- chain fatty acid (SCFA) producing bacteria is associated with decreased future development of bacterial and viral infections 2) We have demonstrated that cell-free DNA (cfDNA) profiling in the blood and in the urine can simultaneously detect changes in the microbiome and virome over time and detect infections in transplant recipients. Importantly, with respect to cfDNA profiling, we have developed a novel technique called SIFT-seq to overcome the challenge of environmental contamination in low biomass specimens. By biochemically tagging the biological specimen prior to downstream DNA isolation, we can bioinformatically remove DNA introduced during sample preparation and accurately identify the microbiome and virome in low biomass specimens.
In this study, we propose to recruit 300 kidney transplant recipients at the time of transplantation for serial fecal, urine, and blood specimen collections during the first year after transplantation. We will profile the gut microbiome using metagenomic sequencing to identify taxa at the species level and using metabolomic SCFA profiling. In addition, we will profile the blood and urine specimens using our novel technique, SIFT-seq, to identify the blood and urine microbiome and virome with high sensitivity and specificity.
In Aim 1, we will determine the fecal bacterial and SCFA profiles that predict the risk of infections in kidney transplant recipients. In Aim 2, we will determine the blood and urine cfDNA profiles that are diagnostic and predictive of infections in kidney transplant recipients.
本网站所有内容来源注明为“梅斯医学”或“MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明来源为“梅斯医学”。其它来源的文章系转载文章,或“梅斯号”自媒体发布的文章,仅系出于传递更多信息之目的,本站仅负责审核内容合规,其内容不代表本站立场,本站不负责内容的准确性和版权。如果存在侵权、或不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言











#肾移植# #美国国立卫生研究院#
60